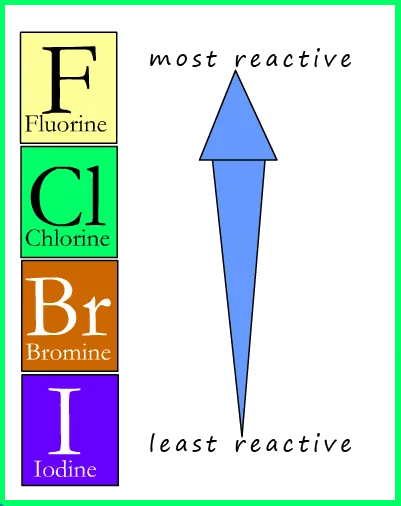
No, bromine is not more reactive than chlorine. In the halogen group, reactivity generally increases as you move up, with fluorine being the most reactive. Chlorine, being higher than bromine in the group, is more reactive due to its smaller atomic size and higher effective nuclear charge.
Bromine, with a larger atomic size, is less reactive than chlorine. Reactivity in the halogen group follows the trend of decreasing as you move down the periodic table. Therefore, chlorine is more reactive than bromine, a fundamental principle in understanding the behavior of elements in the same chemical group.
What Defines Chemical Reactivity?
Chemical reactivity refers to the tendency of a substance to undergo chemical reactions. It is a fundamental concept in chemistry that describes how readily atoms or molecules react with each other, leading to the formation of new compounds. Reactivity can vary widely among different elements and compounds, and it is influenced by various factors.
What factors influence chemical reactivity?
Electron Configuration
Electron configuration refers to the arrangement of electrons in an atom’s electron shells. The distribution of electrons determines the atom’s stability and its likelihood of engaging in chemical reactions.
Atoms with incomplete electron shells are generally more reactive as they seek to achieve a stable configuration through gaining, losing, or sharing electrons.
Alkali metals like sodium have a single electron in their outermost shell, making them highly reactive. Sodium readily loses this electron to achieve a stable configuration.
Atomic Structure
Atomic structure, including factors such as atomic size and effective nuclear charge, plays a crucial role in reactivity. Larger atoms may have electrons that are farther from the nucleus, making it easier for them to be involved in reactions. Effective nuclear charge influences the attraction between electrons and the nucleus, impacting reactivity.
Halogens like fluorine have a smaller atomic size and high effective nuclear charge, making them highly reactive and prone to gaining electrons.
Bonding Characteristics
The type of bonds an element forms (ionic, covalent, metallic) affects its reactivity. Ionic bonds involve the transfer of electrons, covalent bonds involve sharing, and metallic bonds involve a sea of electrons. Different bonding characteristics contribute to how readily an element interacts with others.
Metals tend to have metallic bonding, allowing them to conduct electricity and heat. Nonmetals may form covalent bonds, leading to a different set of properties.
What are the key properties and uses of bromine?

Bromine, with its chemical symbol Br and atomic number 35, is a halogen that shares its group with fluorine, chlorine, iodine, and astatine. It is the only non-metallic element that exists in a liquid state at room temperature, presenting as a reddish-brown liquid.
Discovered in the 19th century, bromine is characterized by its strong, unpleasant odor. It is highly corrosive and has distinctive chemical properties that make it a valuable element in various industries.
The liquid state of bromine at room temperature distinguishes it from its halogen counterparts, and its unique properties make it an essential component in numerous chemical applications.
Physical and Chemical Properties
Physical Properties
Bromine has a density higher than water, causing it to sink. Its liquid form makes it distinctive among the halogens, and it can easily transition between liquid and vapor states.
Bromine exhibits a range of colors, from dark red to brown. Its vapor has a characteristic intense color, contributing to its identification.
Bromine is known for its pungent, unpleasant odor, reminiscent of its Greek origin, “bromos,” meaning stench.
Chemical Properties
Bromine is highly reactive and readily forms compounds with metals and nonmetals. It reacts vigorously with substances like aluminum, forming aluminum bromide.
In its liquid state, bromine is a strong oxidizing agent, participating in redox reactions.
Bromine’s physical and chemical properties showcase its unique characteristics, making it a valuable element in various chemical processes.
Common Uses of Bromine
Flame Retardants
Bromine compounds, such as polybrominated diphenyl ethers (PBDEs), are widely used as flame retardants. They inhibit the ignition and slow the spread of fires in materials like textiles, plastics, and electronics.
Pharmaceuticals
Bromine compounds are integral in the synthesis of pharmaceuticals. Specific brominated compounds are used in medicinal and chemical research.
Water Treatment
Bromine is employed as a disinfectant in water treatment processes, particularly in hot tubs and swimming pools. It serves as an effective alternative to chlorine in certain applications.
The diverse applications of bromine underscore its importance in ensuring safety, functionality, and efficiency in various industries. Its role in flame retardancy and water treatment, as well as in pharmaceuticals, emphasizes its versatility and significance.
What are the characteristics and common applications of chlorine?
Chlorine, with the chemical symbol Cl and atomic number 17, is a halogen belonging to the same group as fluorine, bromine, iodine, and astatine. At room temperature, chlorine is a diatomic gas, appearing greenish-yellow.
It has a distinct, sharp odor, often associated with household bleach. Discovered in the late 18th century, chlorine has played a crucial role in various applications, ranging from water treatment to the synthesis of chemicals.
Chlorine’s gaseous state and distinctive color contribute to its identification, and its odor is well-recognized due to its common use in disinfection processes.
Physical and Chemical Properties
Physical Properties
Chlorine is a diatomic gas under standard conditions, appearing greenish-yellow. Its gaseous nature distinguishes it from its halogen counterparts, which are primarily liquids at room temperature.
Chlorine has a pungent and distinctive odor, reminiscent of household bleach. The odor threshold is low, making it easily detectable even at low concentrations.
Chemical Properties
Chlorine is highly reactive and readily forms compounds with a variety of elements. It is a strong oxidizing agent and can participate in redox reactions.
Chlorine can displace less reactive halogens from their compounds. For example, it can displace iodine from potassium iodide.
Chlorine’s reactivity and distinctive physical properties contribute to its role in various chemical reactions and applications.
Common Uses of Chlorine
Water Treatment
Chlorine is widely used to disinfect water and eliminate harmful microorganisms. It is added to drinking water and swimming pools to ensure public health and prevent the spread of waterborne diseases.
Chemical Synthesis
Chlorine is a key component in the synthesis of various chemicals, including PVC (polyvinyl chloride), solvents, and chlorinated intermediates used in pharmaceuticals and agrochemicals.
Bleaching Agents
Chlorine compounds, such as sodium hypochlorite, are commonly used as bleaching agents in the production of paper, textiles, and cleaning products.
The widespread use of chlorine in water treatment and chemical synthesis underscores its importance in maintaining public health and supporting various industrial processes.
How does bromine exhibit reactivity compared to other halogens?
Bromine exhibits reactivity compared to other halogens based on its position within the halogen group on the periodic table. The halogens include fluorine, chlorine, bromine, iodine, and astatine.
The reactivity of halogens generally increases as you move up the group, with fluorine being the most reactive and astatine being the least reactive.
Electron Configuration
Bromine, like other halogens, has seven electrons in its outermost electron shell, requiring one more electron to achieve a stable, full outer shell (octet). This makes bromine highly reactive as it tends to gain an electron through chemical reactions.
Atomic Size
Bromine has a larger atomic size compared to fluorine and chlorine. The increase in atomic size down the halogen group results in weaker intermolecular forces. While larger atoms generally have a lower reactivity, bromine is more reactive than chlorine due to its larger size.
Effective Nuclear Charge
The effective nuclear charge, which is the net positive charge experienced by an electron in the outermost shell, decreases down the halogen group. This decrease in effective nuclear charge contributes to the trend of increasing reactivity down the group.
Bond Dissociation Energy
Bromine has a lower bond dissociation energy than fluorine but higher than chlorine. Bond dissociation energy is the energy required to break a chemical bond. The lower bond dissociation energy in bromine indicates that it is relatively easier for bromine to undergo chemical reactions and break bonds compared to fluorine.
Displacement Reactions
Bromine can displace less reactive halogens from their compounds. For example, it can displace iodine from potassium iodide in a displacement reaction. This demonstrates that bromine is more reactive than iodine.
What defines the reactivity of chlorine, and how does it compare to other halogens?
The reactivity of chlorine is defined by various factors, and its comparison to other halogens sheds light on its distinctive behavior.
Electron Configuration
With seven electrons in its outermost electron shell, chlorine is just one electron short of achieving a stable, full outer shell. This electron deficiency drives its reactivity, as chlorine readily participates in chemical reactions to gain an electron and achieve a stable electron configuration.
Atomic Size
Chlorine has a relatively small atomic size compared to other halogens. The smaller the atom, the more easily it can approach and react with other atoms or molecules. This smaller size contributes to the heightened reactivity of chlorine.
Effective Nuclear Charge
The effective nuclear charge, representing the net positive charge felt by an electron in the outermost shell, is relatively higher in chlorine compared to larger halogens. This increased charge attracts electrons more strongly, enhancing chlorine’s reactivity.
Bond Dissociation Energy
Chlorine forms strong covalent bonds, and breaking these bonds requires a significant amount of energy. The high bond dissociation energy indicates that chlorine tends to form stable bonds, but when those bonds are broken, a substantial release of energy occurs.
Comparison with Other Halogens
Fluorine: Fluorine, being the smallest and most electronegative halogen, is exceptionally reactive. Its small atomic size and high effective nuclear charge make it highly eager to gain electrons.
Bromine: Bromine has a larger atomic size compared to chlorine, resulting in lower reactivity. While it is more reactive than iodine, it is less reactive than chlorine.
Iodine: Iodine, with a larger atomic size and lower effective nuclear charge, is the least reactive halogen. Its reactivity is lower compared to chlorine and bromine.
Reactions Involving Chlorine
Water Disinfection: Chlorine’s reactivity is harnessed in water treatment, where it undergoes redox reactions to disinfect water by eliminating harmful microorganisms.
Organic Synthesis – Chlorination: Chlorine is involved in organic synthesis through chlorination reactions, where it adds across carbon-carbon double bonds, leading to the synthesis of chlorinated organic compounds.
FAQ
What reacts faster, bromine or chlorine?
Bromine reacts more slowly than chlorine. Chlorine’s smaller size and higher effective nuclear charge make it more reactive, leading to faster reactions compared to bromine.
Why is bromine more reactive?
Bromine is not more reactive; it is less reactive than chlorine. Chlorine’s higher reactivity is due to its smaller atomic size and increased effective nuclear charge, driving its eagerness to engage in chemical reactions.
Is bromine the most reactive?
No, bromine is not the most reactive. Fluorine holds that title within the halogen group due to its small atomic size and high electronegativity.
Why is Cl more electronegative than Br?
Chlorine is more electronegative than bromine due to its smaller atomic size and higher effective nuclear charge, which enhance its ability to attract and hold onto electrons.
Is Br a stronger acid than Cl?
No, bromine is not a stronger acid than chlorine. Both are halogens and do not act as acids in the typical sense; their reactivity is more characteristic of oxidizing agents.
Why is chlorine very reactive?
Chlorine is highly reactive due to its small atomic size, high electronegativity, and the ability to readily gain electrons, making it an effective oxidizing and disinfecting agent.
Is Br more electronegative than Cl?
No, bromine is not more electronegative than chlorine. Chlorine has a higher electronegativity due to its smaller size and increased effective nuclear charge, making it more electron-attracting than bromine.
Final thoughts
Chlorine is more reactive than bromine. This is because chlorine is smaller and more ready to react with other things, while bromine is a bit bigger and not as reactive. Knowing this helps us in chemistry to predict how these elements will act in different situations.
Even though both bromine and chlorine have their own special qualities and uses, chlorine is the one that reacts more within the group of elements they belong to. These differences in how they react helps us understand more about these elements and how they work.