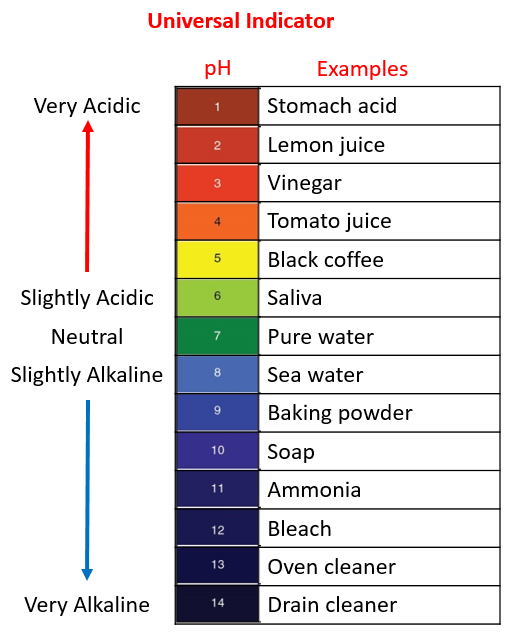
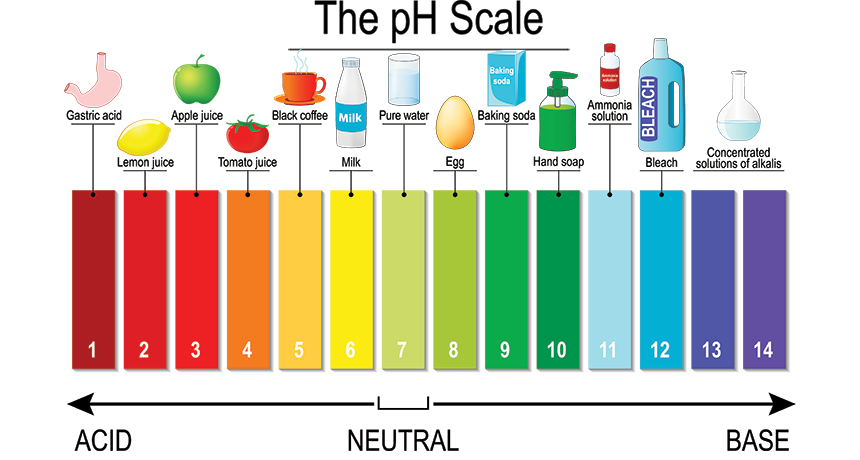
Bleach is an alkali. The chemical formula of bleach is NaOCl, and in an aqueous solution, it dissociates into sodium ions (Na+) and hypochlorite ions (ClO-). This dissociation leads to the release of OH- ions, making the solution basic.
Moreover, the pH value of sodium hypochlorite, the main component of bleach, typically ranges between 11 and 13. Therefore, bleach falls on the alkaline side of the pH scale.
Composition of Bleach

Bleach typically consists of sodium hypochlorite (NaOCl) as its primary active ingredient. This compound is composed of sodium ions (Na+) and hypochlorite ions (ClO-). The sodium hypochlorite is a key component responsible for the disinfectant, bleaching, and cleaning properties of bleach.
Furthermore, the chemical formula NaOCl represents the composition of bleach, and it is crucial in various household applications for its effectiveness in removing stains and sanitizing surfaces.
pH Level of Bleach
The pH level of bleach is typically above 7, indicating that it is alkaline. The main component of bleach, sodium hypochlorite (NaOCl), contributes to its alkalinity. The pH of sodium hypochlorite usually falls in the range of 11 to 13 when in solution. This alkaline nature makes bleach effective for various cleaning and disinfecting purposes, distinguishing it from acidic substances on the pH scale.
Bleach Unveiled: pH and Alkalinity Explained Simply
Acids are known for their sour taste and their ability to turn blue litmus paper red. They release protons or accept unshared pairs of electrons in their aqueous solution, and their pH is below 7 on the scale.
Now, let’s talk about bleach. Is it acidic? No, it’s not. Bleach contains sodium hypochlorite, and it’s not an acid because its pH value is above 7. It doesn’t release protons or accept unshared electrons. In fact, in an aqueous solution, it turns red litmus paper to blue, which is a telltale sign of an alkaline substance.
Also, Breaking it down further, when sodium hypochlorite dissociates into sodium ions (Na+) and hypochlorite ions (ClO-), the sodium ion is neutral, and the hypochlorite ion has an unshared pair of electrons to give away.
Now, let’s look at the pH scale. Solutions with a pH between 1 and 7 are considered acidic, while those between 7 and 14 are alkaline. Sodium hypochlorite, the main component of bleach, is a basic compound with a pH greater than 7.
To figure out the pH of a 0.01 M solution of sodium hypochlorite, we calculate its Kb value. Given that the Ka value is 3 X 10-8, we find Kb to be 3.3 X 10-7. Using this value, we determine the concentration of hydroxide ions, finding a pOH of 3.7. Considering pOH + pH = 14, we get a pH of 10.3, confirming that sodium hypochlorite is indeed basic.
Why Bleach is Alkaline?
There are a few theories that help explain why bleach behaves as a base.
Firstly, there’s the Bronsted-Lowry Theory, which says that a substance acts as a base if it’s ready to soak up protons from another molecule in an aqueous solution.
Then, there’s the Arrhenius Theory, stating that a substance is considered alkaline if it can produce hydroxide ions (OH-) in a solution.
Lastly, the Lewis Theory suggests that a base is a substance with unshared electrons that it can donate to other molecules.
Now, looking at sodium hypochlorite (NaOCl), when it dissociates, it forms Na+ and ClO- ions. The Na+ ion is neutral and doesn’t impact the solution’s nature. However, the hypochlorite ion acts as a proton acceptor in the solution, behaving as an acid according to the Bronsted-Lowry theory. This results in the formation of hydroxide ions, meeting the conditions of the Arrhenius theory, which defines alkalinity.
Moreover, the hypochlorite ion, with its negative charge, indicates unshared electrons. As seen in the reaction, it donates these electrons to the hydrogen ion of the water molecule, aligning with the Lewis theory, and classifying it as a base.
In simpler terms, bleach’s alkaline nature comes from its ability to accept protons, produce hydroxide ions, and donate unshared electrons in certain reactions.
Why Isn’t Bleach Acidic?
Well, according to the three theories we chatted about earlier:
- Bronsted-Lowry Theory: Acids give away protons, but sodium hypochlorite in bleach doesn’t do that. It’s not in the business of giving away protons.
- Arrhenius Theory: It produces hydrogen ions (H+) in a solution, and bleach doesn’t do that either. No hydrogen ions here!
- Lewis Theory: Lastly, it accepts unshared electrons from other molecules. Again, sodium hypochlorite doesn’t fit the bill. It’s not into accepting those unshared electrons.
So, by the rules of these theories, bleach is a no-go for being an acid.
Properties
- Appearance: It looks like a greenish-yellow solid, and when it’s hanging out with water, it comes as a pentahydrate.
- Name Tag: Its chemical name is NaOCl, and it’s a mix of sodium and hypochlorite ions.
- Weight Watcher: Weighing in at 74.442 g/mol, it’s not too heavy.
- Scent Story: It has a sweetish smell, kind of like chlorine.
- Density Dance: If you measure its density, you’ll find it’s 1.11 g/cm3.
- Temperature Tales: It gets a bit melty at 18°C and likes to boil at 101°C.
- Water Buddy: It’s pretty good friends with water, dissolving at 29.3 g/100mL at 0°C.
- pKa and pKb Tag: It has these cool values, pKa is 7.5185, and pKb is 6.4815.
- Stability Status: The dry version can be a bit explosive, so we keep it cool in the fridge as the pentahydrate, which is more chill.
- Oxidizing Olympian: It’s a strong oxidizer and can be a bit of a corrosion champ.
- Metal Mixer: When it hangs out with metals, it might create some hydrogen, and things could get a bit explosive if things heat up.
Sodium Hypochlorite: Versatile Uses in Everyday Life
- Household Hero: Ever used bleach for cleaning at home? That’s sodium hypochlorite, making things squeaky clean. It’s usually mixed with a bit of sodium hydroxide to slow down its break down.
- Stain Fighter: Got stubborn stains on your stuff? Sodium hypochlorite is like a superhero against mold stains, tea or coffee stains, and even dental stains.
- Germ Buster: Need to disinfect a really dirty area? A 0.5% solution of sodium hypochlorite does the trick, wiping out germs.
- Dakin’s Solution: This is a mix of sodium hypochlorite and boric acid, used for different medical purposes.
- Odor Warrior: It fights bad smells by making organic dirt water-soluble and non-smelly. So, it’s like a deodorizer.
- Dental Helper: Dentists use it during surgeries to tackle microbes. It’s like a superhero for your teeth.
- Water Protector: In paper and pulp mills, sodium hypochlorite keeps the water safe by fighting off stuff that shouldn’t be there.
- Cyanide Chaser: Ever heard of cyanide in water? Sodium hypochlorite helps turn that toxic stuff into something non-toxic.
- Chemical Warfare Neutralizer: In serious situations, it helps clean up protective gear used in chemical warfare.
- Skin Soother: In small amounts, it’s used to treat eczema and skin damage from too much sun or radiation therapy.
- Industrial Pal: Industries like textiles and detergents use it in their processes.
- Food Guardian: Ever wondered how food prep equipment is kept clean? Sodium hypochlorite is on the job.
- Pool Cleaner: Ever been to a pool? Chances are sodium hypochlorite is helping keep it crystal clear.
- Oil Refinery Buddy: Even in the big leagues like petroleum refining, sodium hypochlorite plays a part. It’s versatile!
FAQ’s
1. What is the pH of bleach?
Bleach has a pH between 11 and 13, making it highly alkaline and corrosive.
2. Is bleach an alkaline substance?
Yes, bleach is an alkaline substance with a pH level of 11, placing it among common household alkalis.
3. Is bleach a strong or weak acid?
Yes, Bleach is not an acid; it is a strong base, along with examples like lye, with potential skin-damaging properties.
4. Can I mix acid with bleach?
No, mixing bleach with acids, ammonia, or other cleaners can result in serious injuries and is strongly advised against.
5. Why is bleach alkaline?
Sodium hypochlorite, the main component of bleach, is alkaline. Household bleach contains NaOH to increase alkalinity, forming hypochlorous acid and hypochlorite ions.
6. Is toothpaste acid or alkaline?
Toothpaste is slightly alkaline, with an average pH of 8, contributing to its nature as a cleaning agent.
7. Is bleach alkaline or neutral?
Bleach is alkaline, specifically an alkaline solution of sodium hypochlorite, used for cleaning, whitening, and disinfecting.
8. Why is bleach so acidic?
While bleach contains hypochlorous acid, its high alkaline pH (8-13) results in the conversion of most hypochlorous acid to hypochlorite, making it less effective as a disinfectant.
9. What pH is lemon juice?
Lemon juice has a pH between 2 and 3, signifying its acidity, being 10,000–100,000 times more acidic than water.
Final Words
Bleach is unequivocally alkaline. Its primary component, sodium hypochlorite (NaOCl), imparts an alkaline nature to the solution. Contrary to acids, bleach does not release protons, accept unshared electrons, or exhibit characteristics aligning with acid behavior.
In addition, the dissociation of sodium hypochlorite results in the formation of hydroxide ions, contributing to the alkalinity of the solution. This distinction is crucial in understanding bleach’s role as a disinfectant, stain remover, and cleaning agent in various household and industrial applications.