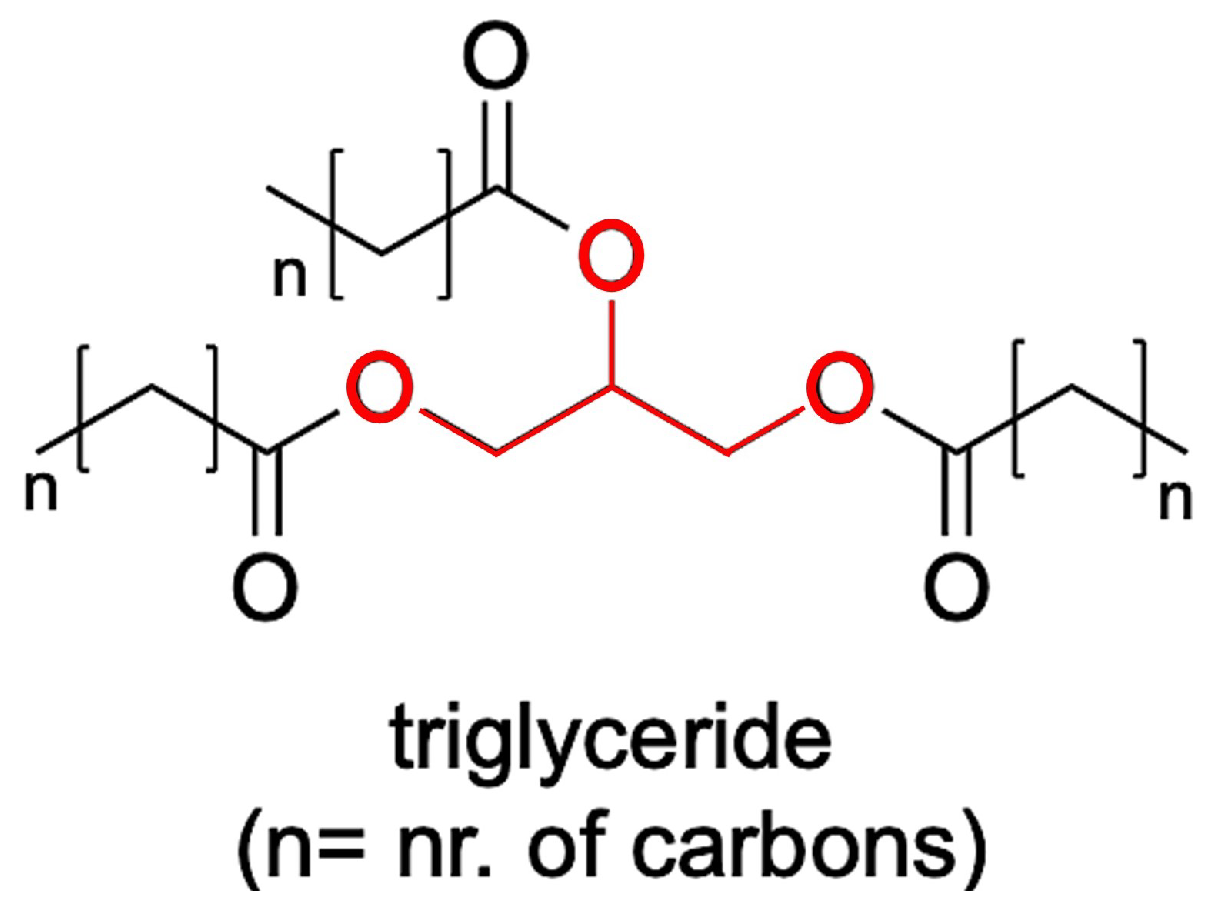
Triglycerides are fundamentally different from polymers due to their unique molecular structure and composition. Polymers are characterized by their large, repeating chains of monomers, forming complex macromolecules.
In contrast, triglycerides are composed of just three fatty acid chains linked to a glycerol molecule. This simplicity sets triglycerides apart.
The distinct arrangement of triglycerides reflects their role in biology as energy storage molecules. They serve as a concentrated source of energy, stored in our adipose tissues, to be released when needed.
Polymers, on the other hand, exhibit a wide diversity of monomers and complex chemical bonds, resulting in materials as varied as DNA, plastics, and proteins. While polymers can have thousands or even millions of monomers, triglycerides are relatively simple in comparison.
This difference in structure and function underscores why triglycerides and polymers are not synonymous in the world of chemistry and biology. Triglycerides serve a unique role as energy reservoirs, while polymers encompass a vast array of molecules with multifaceted functions.
What Are Molecules?
At their core, molecules are groups of atoms bonded together. Atoms, the smallest units of matter, combine to form molecules when they share or exchange electrons. This bonding gives rise to a wide variety of molecules, each with its unique properties.
Molecules in Nature
Molecules are all around us, both in the living and non-living world. Water, for example, is a molecule composed of two hydrogen atoms and one oxygen atom (H2O). This simple molecule is essential for life and has unique properties that make it an excellent solvent and a key component of many biological processes.
Molecules in Biology
In biology, molecules take on even greater importance. DNA, the molecule of heredity, carries genetic information and directs the development and functioning of all living organisms. Proteins, made up of amino acid molecules, are the workhorses of the cell, performing tasks such as catalyzing chemical reactions and providing structural support.
Molecules in Chemistry
In chemistry, understanding the properties and behavior of molecules is central to the field. Chemists study how molecules interact with each other, undergo chemical reactions, and form the basis for the materials we use in everyday life, such as plastics and medicines.
What Are Polymers?
Polymers are a fascinating and versatile class of molecules that play a significant role in our everyday lives. To put it simply, polymers are large molecules made up of smaller units called monomers, which are chemically bonded together in long chains or networks. These monomers can repeat multiple times, creating a macromolecule with a wide range of properties and functions.
The Building Blocks: Monomers
At the heart of every polymer are its monomers. These are the basic, repeating units that link together to form the polymer. Think of monomers as the Lego bricks of the molecular world. Common monomers include molecules like ethylene for polyethylene (used in plastic bags) or amino acids for proteins (essential for life).
Chain Formation: Polymerization
The process of creating a polymer from its monomers is called polymerization. It can be initiated in various ways, such as through heat, pressure, or chemical reactions. During polymerization, monomers chemically bond together, forming long chains. This chain-like structure is what distinguishes polymers from smaller, simpler molecules.
Diverse Applications
Polymers are incredibly diverse and have a vast range of applications. They can be found in everyday items like plastic bottles, rubber tires, and even clothing fibers. Polymers are also used in specialized fields, such as aerospace engineering (for lightweight materials), medicine (for drug delivery systems), and even in DNA, the molecule of heredity.
What Is the Role of Triglycerides in the Body’s Energy Management?
Triglycerides, often referred to as “fats,” are an essential and highly versatile type of molecule found in the human body. While they are often associated with negative connotations due to their role in weight gain and cardiovascular health, triglycerides serve numerous vital functions in our physiology.
The Structure of Triglycerides
At the heart of triglycerides lies a relatively simple yet ingenious structure. Each triglyceride molecule is composed of two main components:
- Glycerol: Glycerol is a small, three-carbon molecule that serves as the backbone of the triglyceride. It is water-soluble and forms the foundation to which fatty acids attached.
- Fatty Acids: Fatty acids are long chains of carbon and hydrogen atoms that vary in length and saturation. These chains, which can be either saturated (no double bonds) or unsaturated (containing double bonds), are what give triglycerides their distinct properties.
The magic happens when three fatty acids bind to a single glycerol molecule, forming a triglyceride. This structure enables triglycerides to store and transport energy efficiently.
Function of Triglycerides
Energy Storage: Triglycerides act as our body’s energy savings account. When we consume more calories than our immediate energy needs, the excess energy is converted into triglycerides and stored in specialized fat cells called adipocytes. Later, when the body requires additional energy, these stored triglycerides are broken down and used as a fuel source.
Insulation and Protection: Triglycerides, especially those stored in adipose tissue, provide insulation to help regulate body temperature. They also serve as a cushion, protecting vital organs from physical trauma.
Transport of Dietary Fats: When we consume fats in our diet, they are initially digested and broken down into fatty acids. These fatty acids are then reassembled into triglycerides in the intestines, allowing for efficient transport of dietary fats to various tissues for energy or storage.
Dietary Sources of Triglycerides
Triglycerides are abundant in many foods, particularly those rich in fats. Common dietary sources of triglycerides include oils (olive oil, vegetable oil), butter, dairy products, nuts, seeds, avocados, and fatty cuts of meat.
Additionally, carbohydrates in our diet can be converted into triglycerides and stored as fat when consumed in excess.
Balancing Triglyceride Levels
While triglycerides are essential for our overall health, excessively high levels of triglycerides in the bloodstream can be a cause for concern. Elevated triglyceride levels are associated with an increased risk of cardiovascular diseases. Lifestyle factors such as diet, physical activity, and genetics can influence triglyceride levels. Maintaining a balanced diet rich in healthy fats, regular exercise, and effective stress management are key strategies to help keep triglyceride levels within a healthy range.
What Are the Diverse Roles of Polymers in Our Daily Lives?
Polymers, often referred to as the “macromolecules of life,” are a fascinating and indispensable class of compounds that warrant a closer examination. These versatile molecules are pivotal in a wide array of applications, spanning from the natural world to the synthetic realm.
The Diversity of Polymers
Polymers exhibit extraordinary diversity, categorized based on their origin and properties
Natural Polymers: These polymers are found in nature and are crucial to life. DNA, the blueprint of all living organisms, is a biological polymer. Proteins, responsible for countless functions in our bodies, are also polymers. Cellulose, a structural component of plant cell walls, is another example.
Synthetic Polymers: Man-made polymers are designed to meet specific needs. Plastics, such as polyethylene and polypropylene, are ubiquitous in modern life, used in everything from packaging to construction. Synthetic rubber, like neoprene and silicone, offers durability and versatility. Synthetic fibers, including nylon and polyester, are widely used in textiles.
Biopolymers: These polymers are of biological origin but are distinct from the polymers found in human-made materials. Starch, a carbohydrate storage molecule in plants, is a biopolymer. Chitin, found in the exoskeletons of insects and crustaceans, is another example. Hyaluronic acid, present in skin and connective tissues, is a biopolymer with significant medical applications.
Applications of Polymers
Polymers are pervasive and find applications in various domains
First, polymers are used to create an array of materials, from lightweight plastics to durable coatings. Their adaptability, ease of processing, and diverse properties make them invaluable in industries such as automotive, aerospace, and construction.
Moreover, biocompatible polymers play a pivotal role in drug delivery systems, medical devices, and tissue engineering. They enable controlled release of medications and can mimic natural tissues, advancing medical treatments and procedures.
However, the flexibility and durability of polymers make them ideal for packaging materials. Plastic containers, films, and wraps help preserve food, reduce waste, and ensure product safety.
Furthermore, polymers are employed in various electronic components, including insulating materials, conductive coatings, and flexible displays, contributing to advancements in modern technology.
What Are The Key Differences Between Triglycerides And Polymers
Triglycerides and polymers are two distinct entities with significant differences in their structures, functions, and roles in various fields.
Chain Length
Triglycerides consist of only three fatty acid chains bonded to a glycerol molecule, resulting in a relatively simple structure.
In contrast, polymers can have much longer chains, often containing thousands or even millions of monomer units. This significant difference in chain length sets them apart at a molecular level.
Composition
Triglycerides are composed of just three types of molecules: glycerol and fatty acids.
Polymers, on the other hand, can have diverse monomer units, leading to a wide range of chemical compositions. This diversity allows polymers to exhibit an extensive array of properties and functions.
Functionality
Triglycerides primarily serve as energy storage molecules and are crucial for insulation and protection.
Polymers, with their various structures and compositions, have an extensive range of functions. They can provide structural support, store genetic information (as in DNA), or form the materials we use daily.
Chemical Bonds
The types of chemical bonds that hold triglycerides and polymers together also differ significantly. Triglycerides are linked by ester bonds, which are a type of covalent bond formed between the glycerol and fatty acid molecules.
Polymers, on the other hand, can form through various types of chemical bonds, such as covalent, ionic, or hydrogen bonds, depending on the specific polymer.
Rigidity vs. Flexibility
Due to their different bonding structures, triglycerides tend to be more flexible. The ester bonds in triglycerides allow for rotational movement between the fatty acid chains, giving them a certain degree of flexibility.
Polymers, on the other hand, can exhibit a wide range of rigidity, depending on the nature of their bonds and the arrangement of monomers. Some polymers can be very rigid and strong, while others can be flexible and elastic.
Biological Roles
In biological contexts, triglycerides primarily function as energy storage molecules and play a role in insulation and protection. Polymers, however, have diverse roles in biology. DNA, a polymer, carries genetic information, while proteins, composed of amino acid polymers, have various functions, including acting as enzymes and providing structural support.
FAQ
Why is a lipid not a polymer?
Lipids are not considered polymers because they lack the repeating monomeric units that define polymers. Instead, lipids, such as triglycerides, have a unique structure with glycerol and fatty acid chains. Polymers, on the other hand, are made up of repeating building blocks, which lipids do not possess.
Why are lipids not polymers but proteins are?
Lipids, including triglycerides, are not polymers because they do not consist of repeating monomeric units. Proteins, however, are polymers made from amino acids, which are the repeating units. This structural difference is why proteins are considered polymers while lipids are not.
Why is triglyceride not a monomer?
Triglycerides are not monomers because they are composed of three fatty acid chains and a glycerol molecule. Monomers are single, smaller units that combine to form larger molecules. Triglycerides do not exhibit this characteristic of monomers.
Are triglycerides a polymer?
No, triglycerides are not considered polymers. They are a type of lipid, and unlike polymers, they do not have repeating monomeric units. Triglycerides have a distinct structure with glycerol and three fatty acid chains.
Why is triglyceride called true fat?
Triglycerides are often referred to as “true fats” because they are the primary form in which fats are stored in the body. They serve as a concentrated source of energy, making them the true storage form of dietary fats.
What is the truth about triglycerides?
Triglycerides are essential molecules in our bodies, serving as an energy source and providing insulation and protection to organs. They are not polymers but unique lipid structures composed of glycerol and fatty acids.
Why triglyceride is called neutral lipids?
Triglycerides are sometimes called “neutral lipids” because they do not have an electric charge. This neutrality distinguishes them from other lipids like phospholipids, which have a charged phosphate group in their structure.
Are true fats also triglycerides?
Yes, “true fats” refer to triglycerides. Triglycerides are the primary form of dietary fats and the primary storage form of energy in the body.
Are triglycerides true biological polymers?
No, triglycerides are not considered true biological polymers. They lack the repeating monomeric units characteristic of polymers. Instead, they have a distinct structure with glycerol and fatty acid chains.
What are triglycerides also known as?
Triglycerides are also known as triacylglycerols or simply “fats.” They are the primary components of dietary fats and play a crucial role in energy storage in the body.
Final words
In the grand world of science, it’s crucial to remember why triglycerides and polymers are not the same. While they may seem similar because they both involve small building blocks, they have distinct roles.
Triglycerides, with their simple structure of three fatty acid chains and a glycerol core, are like our body’s energy reserves and cozy insulation. They keep us warm and provide quick energy when needed.
Polymers, however, are the versatile superheroes of the molecular world. They come in all forms, from DNA to plastic, and serve countless functions, like storing genetic codes or creating everyday objects.
So, the next time you encounter these molecules in your science adventures, keep in mind these key distinctions. It’s these little details that make science exciting, and understanding them helps us appreciate the intricate beauty of the world around us.